OP8 Alt & bis
United States: We started from the title in informals. We haven’t discussed certain OPs. I don’t believe OP8 Alt & Bis are in a position to be finalised. OP8 is dealing with legislative measures to address the problem of designer precursor chemicals. So it’s a sensitive issue because it’s domestic legislation. There are other OPs that could be finalised.
Russian Federation: I just want to flag that for technical reasons, the Russian Federation could not join the informals on this resolution this afternoon due to overlapping developments in other parts of our current Session. We are joining from the capital online only. We weren’t able to come to Vienna like others. Kindly indulge us and not move so quickly in paragraphs that contain new language. It is the first time we see OP8 Alt. Move slowly please.
Chair: We are going to move to our discussions to the following paragraph. They look agreed on informals. Any comments?
OP9 & 10
Chair: Both are agreed in CoW as there are no comments.
OP11
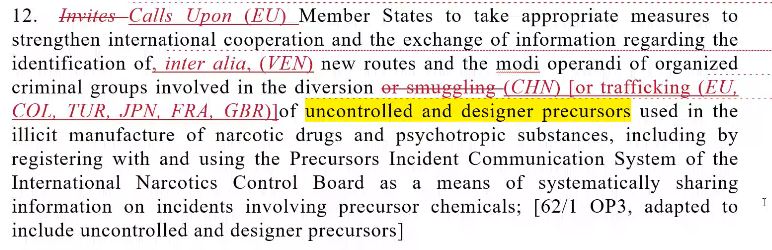
Chair: Two comments here. Nolan – Could you introduce this paragraph?
United States: I can confirm Russia was not in the room for informals. I can assure you we started from the title, so we didn’t get to any OPs. We only discussed PPs in informals. This is not ‘new’. OP11 is very close and we hope to do it in the CoW. They’re discussing tools of the Secretariat of UNODC and INCB to develop responses to the proliferation of these chemicals. One of such tools is PEN Online. There was a suggestion from the EU to welcome new efforts that the INCB is doing on similar systems. The Russian Federation wanted to explore more detail about this new system, potentially with the INCB Secretariat. Have they been able to do it? If they’re comfortable with INCB work, we could move ahead. This was not discussed in informals today. It’s a CoW discussion.
Russia: We are sincerely grateful to the sponsors for understanding the difficulties we had and that we were not at informals and thanks for the explanation that OPs have no new language introduced during informals. On OP11, we don’t have reservations to the whole paragraph but the second part because it’s new language by a delegation on similar system to share information about non-scheduled substances. I hope the INCB Secretariat is at the CoW or sponsors could share, because it sounds like we’re welcoming ongoing efforts of INCB to discuss these issues. Are we inviting INCB to prepare this or is the work ongoing already? Depending on that, we will have comments.
INCB: Indeed, I can confirm INCB is already working on a similar system to the PEN Online. It would function in the same way but would be on a voluntary basis and are substances that are not on Table 1 & 2.
Russia: Thanks to the INCB. It is not customary for our Commission to welcome efforts that have not been completed. Question to the sponsors: maybe we can encourage the INCB to develop a system? Or take note of these efforts? We haven’t seen what this product will look like in the end. We’re generally OK with the explanation provided by the INCB Secretariat.
United States: We’re thinking along the same lines and we’re happy to change to ‘takes note of the efforts’ instead of ‘welcome’ because we have not seen this new system.
Chair: Agreed? Yes, agreed in CoW.
OP12
China: We have proposed in informals that we would like to have “illicit trafficking”. We would like to change the 4th line to “of or illicit trafficking in”.
Chair: There seems to be an agreement on this paragraph. I see no comments, agreed in CoW.
OP13
Chair: There is a similar amendment proposal by China that was already accepted by USA.
Russia: On this paragraph, we received additional comments from our competent authorities. We apologize for not adding them earlier, we only got them today. This language was taken from UNGASS para 3L however it was adapted to non-controlled substances. We flag that the diversion of noncontrolled substances in not a criminal act in Russia, therefore we cannot agree with this paragraph that says, “all drug-related crime”. We propose to delete starting from “to combat…” until “in some cases” and put instead “to prevent and address diversion of or illicit trafficking in”. We are open to using stronger language, but we cannot refer to it as a crime.
USA: We agree to this suggestion. You will see throughout the text the yellow highlight – those are all pertaining to the title that we discussed in informals earlier.
Chair: We can consider this paragraph agreed upon. We will take into consideration the highlighted parts later.
OP14 and 15
USA: Here we are debating how to reflect that competent authorities need training ans assistance in understanding these tools. There were some discussion about complications around Spanish translations of “education…” we will continue finding the right terminology but we are not prepared to agree on this now.
Chair: Okay, then we move on to 15.
Venezuela: We appreciate the USA´s explanation but in fact there are more issues. The original text where this was taken from has more issues and we have some concerns. We are willing to work with the US delegation, but the original text had all these difficult to understand terminologies. We can go along with any word at the beginning – this is not the main concern of this paragraph, we are willing to work with sponsors and those speaking better English than us.
USA: Starting with the beginning of the paragraph, we suggest switching the order at “…INCB to help…” – this is focused on MS action, so we would add “…encourages members states with the assistance of the INCB as appropriate…”. We would take the suggestion “… to provide adequate training to…”. The intent of this is to focus on MS action and in particular practices between MS. Our intent was to invite competent authorities to provide information they deem is appropriate to trusted stakeholders in the chemical industry to educate about legal and regulatory requirements at destination countries. The competent authorities don’t need to educate themselves; they are aiming at those who are transited or are destination countries. This is not to litigate a conversation about source and destination.
Chair: Okay we are now talking about adequate training and not education. That is helpful for Spanish speakers.
Iran: We have observations regarding “providing education”… it should be done with the help of INCB. For the sake of consensus, we are prepared to go along with this verbiage.
Turkey: Sorry for interrupting. We would only emphasise that we prefer ‘source’ and ‘countries’ in the text. Thank you.
Venezuela: Regarding the first part of the paragraph we can agree.
Chair: We advanced slightly. No changes on the second part. What about the second part, colleagues from the US? I see many comments. What do you think?
United States: As we reflect on the comments specifically, I’m seeing a suggestion to include ‘of other member states’ as well as ‘source’ and ‘countries’. It is our feeling that we explained why we did not include the word ‘source’. I remember mentions to remove everything after ‘Member States’. If that option is still supported, we’re happy to end it after ‘of other Member States’.
Chair: Comments?
European Union: Just for the record, we can agree with stopping after ‘Member States’. Retaining the original language, we can agree with the new version on screen and it reconciles all views.
Chair: Agreed in CoW?
Venezuela: I had a comment on the ‘trusted’ relevant stakeholders. Everybody I am going to give information to will be trusted. Can we remove the word ‘trusted’? We already said ‘relevant’. We are concerned saying ‘trusted’ implies we know what the stakeholder will do with this information.
United States: We can remove ‘trusted’. This might be unique to our or other countries’ regulatory systems.
Colombia: Regarding this last amendment. Taking into consideration the consensus of the language in front of us, we just want to confirm to native speakers in English about the language at the end of the paragraph: ‘of their legal and regulatory requirements of other member states’.
United States: The extra ‘their’ was a suggestion of Mexico. It can be removed and changed to ‘of the legal and regulatory’.
Chair: Agreed in CoW? Yes.
OP14bis
Colombia: We are confused. We see three operative paragraphs requesting something from UNODC. 14bis is the same 16bis, same language. Why reiterate? These two paragraphs proposed by Iran contain a very difficult expression for us ‘an impediment’. What is the purpose of the word ‘impediment’ which does not exist in previous resolutions. We consider it more appropriate to work on the original wording of OP16.
United States: We propose skipping OP14 and going to OP15.
OP15
Colombia: We just want to request to incorporate our request to delete 14bis and 15bis that we requested earlier.
USA: We are talking about tools, specifically referring to the UN toolkit on synthetic drugs. There was reference whether the WHO should be involved – by Russia and Turkey. In this case we are not talking about the conventions, we are talking about the toolkit and that merits a reference to WHO. Can we indulge and maintain the reference here?
Russia: My delegation raised a question to the sponsors about what role WHO could play regarding non-scheduled substances. This is a very specific and narrow work. We are sure the WHO will be an active contributor, but this current paragraph specifies a specific part of the toolkit about precursors. We have never added a specific role to WHO, so we are a bit concerned about. We understand we are not talking about a treaty mandated role but the role in general of the WHO. Who is mainly focused on public health. Non-scheduled chemicals have no licit uses in many instances, so they don’t have medical use and that is why we are not clear about what role we are giving to this body.
USA: I am happy to provide additional explanation. The WHO has indeed a very clear role and responsibility. In our view this can include precursors, so when WHO promotes access to availability, that may indeed include substances that can be used in the illicit manufacture of substances as well, so we should not shut this option out because it can negative impact our collective measures. I am sure my colleague will be able to provide instances, but I hope this is a satisfactory explanation.
Chair: Is it possible to remove the reservation of the Russian delegation?
Russia: Thank you for this explanation, we need more information on precursors with medical benefits. We believe that if we have to consider the role of this body, then we might need to work on the language of the paragraph further. Therefore, we ask to go to informals to find solutions.
Chair: Okay.
OP17
Chair: Approved in CoW. We move on to the title.
Title
USA: We had the opportunity to revisit the title in informals. The sponsor offered to work off the proposal of China.
Chair: I see no comments on the title. Can we approve? Agreed in CoW. We finish the consideration for today – oh, China?
China: We still have some reservations… Diversion and illicit trafficking are key problems, so we think it is more appropriate to use “non-scheduled and designer precursors”.
Chair: Lets go back to our remaining issues in informals. I am sure the few remaining difficulties will be solved soon.
USA: It is our strong view that we discussed the title in detail already so we are hesitant to go back to discussing what has already been agreed on. To demonstrate maximum flexibility, if this is what the room wishes… (…)
China: I am sorry that this morning we had a consensus, but I have explained I did not have my colleague with me there who is from the technical department and might have different views as me, as I am from the policy department.