Supporting documents:
MORNING SESSION
Chair: On the request of the extended bureau all submitted questions are published on the website of the commission and a live webcast is also accessible here.
Agenda item 1. We will not spend much time on this today as we have thematic sessions the next month. Each regional group has been invited to nominate experts on the panels during these coming intersessionals. The UNODC research and trend analysis branch will brief the commission on the progress made in the strengthening and streamlining the existing annual report to aid our implementation of all our commitments. Following the resolution 2011/259, the Reconvened 62nd Session, on 12th December, will be devoted to the remaining items on agenda including implementation of international drug control treaties. As per Resolution 58/10, the WHO will orally report in December on their October meeting. This will enable MS to decide on actions to be taken on the recommendations made by the WHO ECDD during the 63rd session of the CND next March. The office of Rapporteur became vacant and the group of Latin American and Caribbean States nominated the Third Secretary of Peru. I open the floor for comments. I see none.
The officers for the Bureau will be elected as follows:
- Chair: Asia Pacific group,
- 1st Vice Chair: Eastern European group,
- 2nd Vice Chair: Western European and Others group,
- 3rd Vice Chair: Latin American and Caribbean states,
- Rapporteur: Africa group
Please, inform about nomination as soon as possible so we can elect on the opening of the 63rd session.
Now we proceed with the preparation to the consideration of proposed scheduling and will follow the same procedure as during the 4th intersessional meeting. The Secretary will elaborate.
Secretary: The written submission by WHO relates to the questions that were received by the 19th of August, as we requested. We received further questions by Japan, Argentina and China which are included in the overall consolidation but aren’t covered in the written responses.
WHO: We appreciate this opportunity to further discuss our recommendations. We thank Member States for submitting questions and showing up today which shows us your immense interest. WHO is mandated by the conventions to issue scientific recommendations to CND whether substances should be places under international control and the level of such control. For this, we work through an independent committee of experts. The work of the ECDD is scientifically robust – it assesses the risks of abuse, dependence and harm to health while taking into account the importance of therapeutic use where relevant. We will not look at economic consequences, we are focused on the aspects of the mandate. The publications and reports used for our analysis are available on our website. We use data from UN sister agencies, regional organisations and, increasingly, from Member States. To make this information transparent, we have established a website. What are the recommendations for? They seek to prevent harms and make sure access is enabled to medically useful substances. The level of international control should be considered as a minimum requirement – it is at the discretion of Member States to implement more stringent regulations.
Why have we embarked on this review? First of all, we are complying to our mandate and CND request. The process did not happen overnight; it has been a process that started more actively in 2014, when information became more available both with respect to harms and therapeutic use. We received a request from a number of countries to engage more intensively and conduct independent assessments as they were seeing a wider promotion of medical cannabis, among other reasons. Cannabis has never been subject to a formal review by the WHO ECDD, so it was timely.
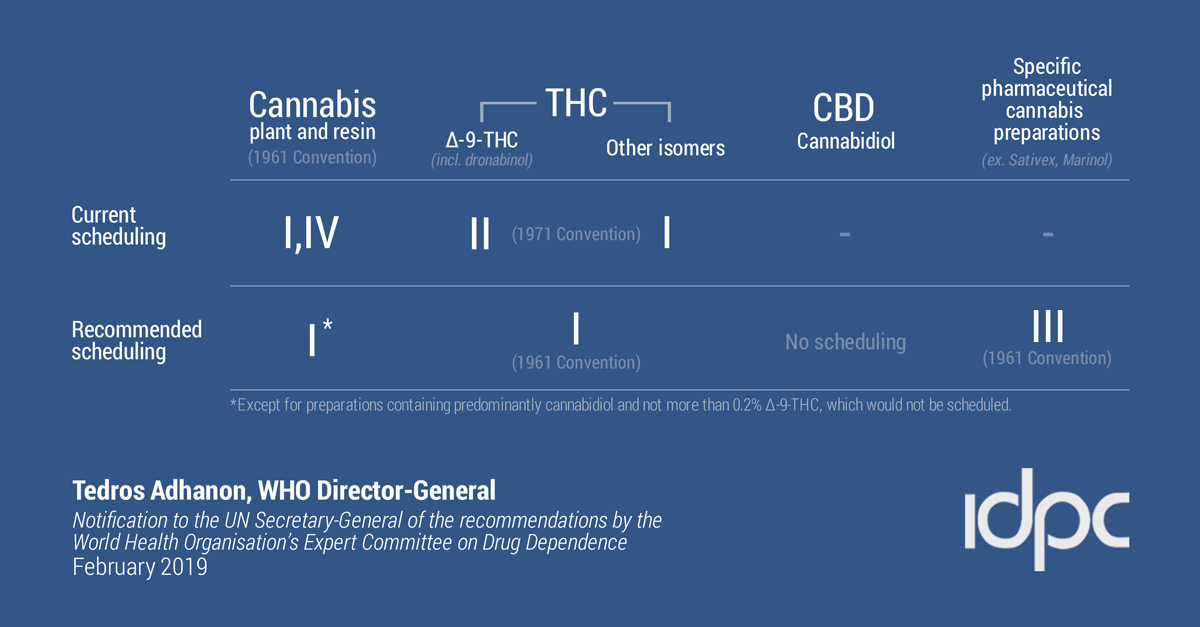
Cannabis and cannabis resin: Currently schedule I and 4 of 1961 convention.
Recommendation: Delete from schedule 4.
Rationale: Cannabis is not similar to other schedule 4 substances, it has proven medically useful.
Dronabinol (delta-9-thc): Currently schedule II of 1971 convention.
Recommendation: add to schedule 1 of 1961 convention, delete from the 1971 convention.
Rationale: Delta-9-thc was not known to be the main active component at the time of original scheduling and so placing cannabis and its active component in the same convention schedule makes sense.
Isomers of THC: Currently in the schedule I of 1971 convention.
Recommendation: add to schedule 1 of 1961 convention and delete from 1971 convention.
Rationale: These are not easily differentiated from delta-9-thc, placing them will facilitate international control.
Extracts and tinctures: Currently in schedule I of 1961 convention.
Recommendation: Delete from scheduling.
Rationale: It was scheduled originally because the original active component was not known but it is covered now as per previous recommendations.
Cannabidiol preparations:
Currently schedule I and IV of 1961 convention. Synthetic cannabidiol is not controlled.
Recommendation: Add a footnote to schedule I of 1961.
Rationale: medical use in childhood epilepsy, not psychoactive, no evidence for dependence or abuse. However, trace amounts of THC can be detected – there is a percentage of THC that will be allowed to be contained in CBD products so the proposed change will allow pharmaceutical preparation.
Dronabinol preparations: Currently schedule II of 1971 convention (and I and IV of 1961 as preparation).
Recommendation: add to schedule III of the 1961 convention.
Rationale: not liable for abuse when taken orally, lower level of control will increase access to pharmaceutical preparations while protecting from harm.
Thank you.
Chair: Now we move on to the Q&A.
WHO / ECDD: Some of the questions go beyond the scope of the mandate of the ECDD although they touch on very important issues. We hope to continue the discussion. We responded to all the questions that addressed the focus of our work and so about the rationale and clarification regarding certain recommendations. All the questions and answers as of 10 September can be found here.
Chair: Thank you. I open the floor for general statements and questions – Please stick to the topic.
European Union (EU): Before going into the substance, we welcome this opportunity to accelerate our commitments. It is key to follow-up on implementations. The EU and its Member States welcomes the broad consensus that the 3 conventions and human rights documents are cornerstones and we acknowledge the mandate of WHO, ECDD and UNODC. We are committed to properly evaluate the recommendations ahead of the decision to be taken. We are grateful the answers and explanation and we welcome the contributions of INCB. We still require some clarifications so we welcome the opportunity to further discuss the questions of MS on which we appreciate the input from treaty affairs. We believe the future vote once the impact of the rescheduling has been assessed. We have no doubt that today will provide clarity in that regard.
Mexico: We appreciate this open dialogue and we recognise the level and quality of submitted questions. Various issues might seem obvious but are worthy of re-stating. The dialogue has been evolving reflecting the profound preoccupation our governments have with the safety and well-being of our populations as well as our dedication to science which didn’t exist 50 years ago. We are going into unfamiliar territories but the advancements that we have seen until today allow us to focus on issues that need further clarification. We have taken note of the EU’s petition about the necessity to bring more precision to some terms and the advantage of a glossary. About ECDD redrafting the recommendation, we believe that the commission’s role is to adapt them including as necessary the amendments. By the next meeting, we hope to be in the position to evaluate the advancements and challenges in implementing the UNGASS operational recommendations. We give great value to the close collaboration between INCB, WHO, CND and all Member States. What could be the concrete positive impact of these recommendations to facilitate scientific research into cannabis and its components?
WHO / ECDD: We are keen to support ongoing scientific research; it can increase our knowledge. The practices are very much up to individual countries. In some Member States the rules are relatively restrictive regarding cannabis research. It is the case that in moving cannabis to only Schedule I means less encouragement for strict control. It is for each country to decide how to facilitate or regulate research.
WHO / ECDD: In response to the EU’s submitted question regarding Cannabis and resin, our definitions are based on the 1961 convention and our processes can be found on our website in detail. [The full WHO-ECDD response will be uploaded shortly]
Nigeria: I want acknowledge what has been said by the WHO this morning. The list of publications has been posted on the website which means we can read those to get a better understanding of the issue. The statement regarding the minimum requirement, we think is an acknowledgement of the seriousness of the threat this substance can have on public health. If we are creating a situation where states determine regulations independently, we are applying domestic responses to a global challenge. The risks posed vary country by country but the dangerous effects are evident and they don’t respect borders. There seems to be a lack of clarity in the responses – on the one hand, WHO acknowledges the risks but they also talk about therapeutic use. As INCB has also stated, cannabis is never a first line medication. Is there assessment of the harmful effects of the therapeutic preparations? The benefits of use versus the risk of cannabis have been said to be beyond the WHO’s mandate. We think this is very important so we would like further clarification on this comparison. Our decision will have a serious impact on our future. The time we are spending to try and understand the recommendations is important before we are called upon to take the next step.
Mexico: In the written questions, the United States made a reference to an intervention made by Mexico so we would like to reiterate that the classification of the whole plant as is the case with poppy is a central issue for us. We support the questions addressing the initial cannabis decision during the drafting of the convention. We think that based on practice and convenience, bearing in mind the complexity of the recommendation, CND should address them individually especially the ones that are not interlinked. USA also posed a question regarding fibres and seeds – we would confirmation that initial scheduling was based on lesser degree of knowledge.
Colombia: We still have doubts about the recommendations. Having in mind the definition for cannabis resin from the Single Convention on Narcotic Drugs, and its critical review report from the 41st ECDD meeting, is the term “cannabis resin” only applicable when it is obtained from cannabis plants without any solvent?
WHO / ECDD: In response to Nigeria – The committee considered evidence of harmful effects of therapeutic use. There are clinical trials and extensive evidence regarding the benefits. As with any medications, side effects exist. The therapeutic use has particularly mild side effects, particularly in the case of cannabidiol. Cost and benefits judgements are not within our mandate. WHO is willing to engage in discussion but that is probably more appropriate for an other forum. Mexico also touched on something that is outside of our mandate.
Colombia asked about resin – it is produced naturally by the plant; it does not require a solvent therefore it is not considered an extract or tincture as it is essentially part of cannabis.
Russia: Is there anything unique that other medications can’t address eg. nausea? Cannabis is the most abused drug in the World. Is there data on the risk-benefit ratio? We appreciate the list of publications made public.
United States: We would like to follow-up on the responses given to our submitted questions. The definition in the single convention separates resin, so the confusion is about “purified” as stated in the convention. We also see some inconsistency regarding 0.2% THC content and its explanation. We realize this requires a bit longer response so we can take that later. With regards to the issue that Mexico raised, we do understand that the ECDD took into consideration that THC’s psychoactive properties were not known by 1961, but they were indeed by 1971. So the UNODC, as keeper of the records, they might reveal the rationale behind the first decision. The explanation regarding preparation, we wonder how we would convey the idea when it comes to voting?
Singapore: We should keep in mind our overall goal, the health and welfare of mankind when we take these recommendations into consideration. The removal from Schedule IV comes with a high signature. We think this decision could be misleading for the public. We have a thin line between global, national or international public. Cannabis is more dangerous than we thought. We appreciate the ECDD’s detailed work but we note that research shows Cannabis si not more liable for abuse than other Schedule I substances – we would like the research on this to be made available. Does current scheduling really prohibit necessary research? Would there be a difference after the implementation of the recommendation? Are there real differences between Schedule I and IV? We have waited decades for this process, so I agree with my Nigerian colleague that we need due time to consider the issue and make a sound decision.
WHO / ECDD: Russia asked about the uniqueness of therapeutic use. There is indication that for most use there are other medication available but it has a different way of working in the body from those medication so there are patients who are able to benefit only from that effects. A number of medical reviews showed that a number of patients respond to cannabis who had no success with any other medication. Particular conditions are treated with cannabis so regarding the risk-benefit question, these would have individual answers – publication list.
In response to the US, normally, crude means it has been obtained from the plant so a crude resin possibly contains other parts of the plant and there are no efforts to separate them. There are a variety of methods to separate them but when a solvent is used, it becomes an extract. Purified resin might have been produced by heat and mechanical processed.
The other questions were suggested for another time, but Singapore asked about research. The findings on which we based our decision are described in the critical review and the findings can be accessed through the summaries. There isn’t a prohibition of scientific research – as I indicated earlier, the regulation of such is up to Member States. The information varies from country to country; in some, the current scheduling doesn’t affect the research but, in some others, there seem to be great barriers due to the current scheduling.
WHO / ECDD: [The full WHO-ECDD response will be uploaded shortly]
United States: We understand that trace amounts of THC would be unlikely to be considered as controlled and I think our domestic practice is different. The commentary addresses this issue saying that when a preparation contains more than one substance, it needs to be controlled by the level of the higher control.
ECDD: We had two questions in 5.3 referring to isomers of THC. These isomers do not occur naturally and are not used medically or non-medically. In answering to the United States, there is sufficient evidence to allow comparisons to synthetic cannabinoids, which are not necessary to be moved.
As for 5.4 regarding extracts and tinctures, there are three main types of illicit products derived from the plant: (1) extracts using a solvent, (2) tinctures obtained using alcohol as a solvent and (3) derivatives without the use of a solvent. [The full WHO-ECDD response will be uploaded shortly]
Colombia: How can extracts and tinctures can be recognised as liquid or solid if the plant needs to be destroyed to produce? What is the practical difference between these classifications that has been described by WHO? If 5.2.1, 5.2.2, 5.3.1 and 5.3.2 are approved? If Member States have different interpretations, how can this impact our harmonious cooperation?
United States: In the general explanation provided this morning that by removing extracts and tinctures we would remove some misunderstandings about preparations. Could the ECDD elaborate on that?
China: We were not able to submit our questions in time. We received out question from capital just yesterday. Hong Kong would like to know that WHO would not like to decrease drug control. Cannabis is covered in the 1961 and the definition offered now seems confusing in context with WHO’s classifications. Could you please clarify?
WHO / ECDD: Colombia suggested that making a tincture or extract, cannabis is destroyed. This is not how we consider it, the plant is present in the preparation; it is the preparation of the plant; there are elements of the plant in the product. Cannabis and cannabis resin are equally controlled and the proposed changes don’t change that. It is impossible to tell if a preparation is made from cannabis or from resin. If there is cannabis present, there is resin present. Regarding THC preparations, I don’t exactly recall the query and I would like to follow-up on this and the remaining question later but I would like you to submit them in writing because it was difficult to comprehend this time.
The US asked about products that are neither extracts nor tinctures. Applying heat belongs to an illicit preparation. IF a solvent has been applied, it is an extract. If a solvent hasn’t been applied, it is not.
As for China, extracts and tinctures are a subset of preparations that use solvents; butane hash oil is an example.
Singapore: […]
WHO / ECDD: I believe that is a question for the INCB.
WHO / ECDD: 5.5 regarding Cannabis preparations. Cannabidiol (CBD) is a substance that can be synthesised or obtained from the cannabis plant. When obtained from the plant, under current regulations, it is controlled both as a preparation of cannabis (Schedules I & IV) and as an extract or tincture (Schedule I). Cannabidiol shows no potential for abuse or dependence and any ill-effects are minimal. It is no similar to any other substance controlled under the 1961 Convention. Cannabidiol does have effects on the brain, but like many other substances with such effects, it is not considered psychoactive as it has no significant effects on mental state. Based on this evidence, and its value as a medicine, the Committee considered that cannabidiol should not be controlled under the 1961 Convention. The Committee considered the option of including preparations of cannabidiol in Schedule III of the 1961 Convention. However, that Schedule is for drugs that are controlled and that satisfy the criteria for control. Cannabidiol does not satisfy those criteria. Inclusion in Schedule III lessens the degree of international control but a number of controls are still required. Inclusion of cannabidiol preparations in Schedule III would mean that controls would be required for preparation of a drug that did not satisfy the criteria for inclusion in the schedules of the 1961 Convention. The option of a footnote was adopted after recognition of the precedents of exclusion of dextromethorphan and dextrorphan from control by this means. When produced from the plant (as is the case with the cannabidiol medicine approved in the US and submitted for approval in other countries), cannabidiol preparations will contain trace amount of THC as well as other cannabinoids and non-cannabinoid plant substances. The Committee considered that most of the preparation should be CBD, and no more than 0.2% THC (by weight). The word predominantly was used to describe the proportion of CBD and this was intended to mean that almost all of the content was CBD. The Committee considered that the percentage of CBD to be used in practice could be left to individual Member States in consultation with INCB. The value of 0.2% for THC was specified as WHO had requests from Member States to indicate what maximum percentage was considered appropriate and to ensure that the currently registered CBD medication was exempted from control. That medication has a THC content not greater than 0.15% by weight as a proportion of the total weight of plant material. The Committee also acknowledged that chemical analysis of ∆9-THC to an accuracy of 0.15% may be difficult for some Member States and hence ECDD adopted a limit of 0.2%. On the basis of the Committee’s recommendation, even for a maximum adult dose of CBD, the level of THC (max. 0.2%) will be below the level that would produce significant effects. Cannabidiol preparations for medical use include preparations with a pre-marketing authorisation and could also include magistral preparations executed in pharmacies, if authorised in countries.The Committee was aware that CBD products, such as foods, are being sold in many countries. While CBD does not satisfy the criteria for control under the 1961 or 1971 Conventions, Member States can regulate its availability using their own national legislation. Both THC and CBD are present in the plant in acid form (THCA and CBDA). The acids of each are converted to THC and CBD, respectively, by heat and/or ultraviolet light. Thus, any product that contained predominantly CBD would not contain significant amount of THCA. There are no implications for the control of cannabis plants or hemp plants arising from this recommendation. With regard to other cannabinoids that may be devoid of psychoactive effects e.g. cannabidavarin (CBDV), the Committee considered that each should be considered separately. While there are such substances under investigation for potential therapeutic benefits, this research is in very early stages. With regard to the conversion of CBD to THC mentioned (Japan), this method was described in a scientific paper over 50 years ago (Gaoni, Y. and R. Mechoulam, Hashish-VII. The isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron Vol. 22. 1966. 1481-1488) and has been subject of a patent application. The method is not simple and requires access to a number of chemicals, including certain acids and solvents. The yield is also uncertain, as are the by-products and their side-effects. This would be an expensive and potentially risky method of obtaining THC compared to use of cannabis and hence it is extremely unlikely that it would be implemented. When produced from the plant, cannabidiol prearations will contain trace amounts of THC as well as other cannabinoids and non-cannabinoid plant substances. Evidence from clinical trials conducted with a product containing no more than 0.15% delta-9-THC as a proportion of the total mass from the cannabis plant showed that this did not produce characteristics or effects similar to cannabis. For Member States to control preparations that contain up to 0.15% delta-9-THC as a proportion of the total mass from the cannabis plant, the Expert Committee recognised the difficulty in measurement to this high degree of accuracy (0.15%) and therefore adopted 0.2% as a more reliable measure that would allow Member States to control. The value of 9.2% for delta-9 THC was specified as Who had requests from Member States to indicate what maximum percentage was considered appropriate and to ensure that the currently registered CBD medication was exempted from control. Epidiolex is the brand name for the cannabidiol preparation that has been approved in the US and contains 0.15% of delta-9-THC, as indicated in the patent, as a total proportion of delta-9-THC relative to the entire plant content and expressed by weight. Therefore, the ECDD’s report expressed its threshold of 0.2% delta-9-THC as a proportion of the entire plant content. It is important to note that the amount of delta-9-THC as a proportion of the total weight of the finished product (w/w of the finished product), will be much lower as a result of the addition of excipients to the cannabis plant extract. However, and in order to prevent confusions, and as other manufacturers may in the future use different amounts or types of excipients, it is important to specify the delta-9-THC content relative to the entire plant content by weight which includes CBD and other cannabis compounds. With regard to the conversion of CBD to THC mentioned, this method was described in a scientific paper over 50 years ago (Gaoni, Y. and R. Mechoulam, Hashish-VII. The isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron Vol. 22. 1966. 1481-1488) and has been subject of a patent application. The method is not simple, the yield is uncertain, as are the byproducts and their side-effects. There have been no published reports that this method has been used illicitly for the production of THC.
European Union: We have received additional notes from our Member States that express it is not fully clear why an extensive exception is needed. It could be a barrier. Consumption of cannabis is purely a domestic matter.
Norway: We are still a bit confused because we see this threshold but in Norway, we are thinking that traces of THC and seeds or fibres are also an issue. I see ECDD doesn’t consider them for national control?
United states: I heard that the percentage refers to [on the basis of] dry-weight material but this is not clear in the text. Since Schedule 3 is for drugs that are otherwise controlled, we think this is not appropriate. Introducing a footnote […] are we in effect creating a new schedule? Echoing the EU, our conventions are such that we add substances and if something is not included, it is not controlled. By creating a footnote, we are working outside of that. CBD products extracted from cannabis plants that are produced for horticultural reasons, does this apply to THC in regards to plants that are cultivated for industrial purposes? How are we to determine the original intent? If there is a preparation that meets the criteria of the footnote and also schedule 3, would the footnote prevail because it describes more detail – if something falls under multiple controls, the stricter prevails, so it seems Schedule III would be more appropriate. Do we have guidance on how to proceed in this case?
WHO / ECDD: The intent was to make it clear that cannabidiol is not a substance that satisfies the criteria for control under the conventions. Currently it is not produced without control, it could be interpreted to be subject for control if included in the footnote. We wanted to make it clear that cannabidiol should not be controlled under the conventions.
With regards to seeds and fibers, those are excluded from the conventions and so are actually not relevant to our recommendations.
Some of the USA’s question need to be taken under further consideration and might not be in the WHO mandate. Cannabidiol is produced from a plant, if the intent of the cultivation doesn’t exhaust the criteria, control is not needed. USA’s further questions need to be addressed to INCB.
Mexico: New cannabis products constantly appear on the market without containing any THC whatsoever. We don’t celebrate arbitrarily setting a global threshold at 0.2% when there are other products, such as cacao or black pepper, that naturally contain THC and are not controlled. I also would like to make a note that the topic of mandates of the WHO vs INCB are very relevant for us.
United Kingdom: We have some concerns – Consuming a large amount of CBD would enable consumption of a larger amount of THC. Has the WHO looked into at what level and what dosage does could this impact for example driving?
Russia: Cannabidiol doesn’t satisfy conditions for control but it could be converted into one, so could we consider it a precursor?
WHO / ECDD: Regarding other plants, our understanding is that Cannabis is the only controlled plant containing these substances. If there are other plants brought to our attention, we can consider their assessment.
As for the UK, we reviewed the evidence concerning the doses of THC and the threshold for impaired driving and other ill-effects were taken into account. We noted that consuming such a large dose of CBD would make a person feel extremely unwell and so it is highly unlikely for anyone to consume as much.
The conversion of CBD, as Russia pointed out, is possible to THC but these require significant chemical knowledge, equipment and willingness. If the substance is convertible into a controlled substance, the convention makes it clear that it needs to be scheduled. However, as per the conventions, the method has to be relatively easy. This method is cumbersome and expensive.
WHO / ECDD: Preparations in Schedule II of drugs controlled in Schedule I or Schedule II of the 1961 Convention are exempted from some of the requirements for control of those drugs. However, they are still subject to a significant level of control. Article 2 para 3 of the 1961 Single Convention states: Preparations in Schedule III are subject to the same measures of control as preparations containing drugs in Schedule II, except that article 31, paragraphs 1 (b) and 3 to 15 and, as regards their acquisition and retail distribution, article 34, paragraph (b), need not apply, and that for the purpose of estimates (article 19) and statistics (article 20,) the information required shall be restricted to the quantities of drugs used in the manufacture of such preparations. This makes clear that the exemption for Schedule II products is for some of the requirements only, and not an exemption from control. The Committee considered the evidence regarding pharmaceutical preparations, including Sativex®. Based on conventional usage of the term, pharmaceutical preparations are those that are used for defined medical purposes and therefore that are in dosage forms appropriate for such medical use. These pharmaceutical preparations encompass the ones requiring pre-market approval and the ones produced extemporaneously according to a prescription and to agreed good manufacturing practices. It was considered that individual Member States will have their won criteria for assessing whether a product is for medical use and as addressed in their national legislation. The evidence from medical use of these preparations shows that they were not associated with abuse or dependence. This recommendation is till relevant if the recommendation to move dronabinol (delta-9-THC) to the 1961 Convention is not supported, as the medications may contain dronabinol derived from the cannabis plant and therefore qualify as preparations of cannabis. As they would therefore be subject to control under the 1961 Convention, inclusion of the pharmaceutical preparations in Schedule II is still appropriate. The pharmaceutical preparations recommended to be placed under Schedule III have dronabinol as the active ingredient and the recommended dosage will vary according to factors such as the conditions being treated and patient history. “Pharmaceutical preparations” refers to substances that are intended for medical use and that are, therefore, in dosage forms appropriate for such medical use. These pharmaceutical preparations encompass the ones requiring pre-market approval and the ones produced extemporaneously according to a prescription and to agreed food manufacturing practices. It was considered that individual Member States will have their won criteria for assessing whether a product is for medical use. The evidence from medical use of these preparations showed that they were not associated with abuse or dependence. With respect to the statement referred to above [by Mexico], “There is no difference in the therapeutic effects or adverse effects of synthetic ∆9-THC compared to ∆9-THC from the Cannabis plant”, it should be noted that dronabinol is the international non-proprietary name for (-)-∆9- THC, whether it is found naturally in the cannabis plant or produced synthetically. Delta-9 THC pharmaceutical preparations are typically consumer through oral administration. Placement of pharmaceutical preparations of cannabis and dronabinol in Schedule III would require that delta-9-THC is not readily recoverable, which means that it cannot be used as a vapor inhalation method or smoking method as other non-medical delta-9 THC preparations described. With respect to the question on comparing the benefits of research and utilization of preparations of cannabis versus their risks, it is beyond the mandate of the ECDD to make this comparison. However, under section 5.1 in this document, it is said that the ECDD recommended to maintain cannabis and cannabis preparations under Schedule I because of similar abuse potential to cannabis and similar ill effects as other substances under schedule I. ECDD also acknowledges the recognised scientific evidence for therapeutic use of cannabis preparations in important conditions such as the management of pain and of muscle spasticity in multiple sclerosis. In all areas of public health, research that is based on robust scientific evidence is needed to ensure better health and wellbeing for people, in particular the most vulnerable. Scientific research on the use of cannabis is no exception and there are currently several hundreds of clinical trial that are being performed to explore efficacy and safety profiles of cannabis for therapeutic use.
Iran: About pharmaceutical preparations, we have an observation: There is no reference on purity – we think there is a need for a footnote. We should notice other limitations such as the prescription dose for children and adolescent as well as incidental intoxication as Cannabis is the most popular substance in this age-group. We need strong evidence and additional research before moving to Schedule III. Is the recommendation only about delta-9-thc taken orally? What kind of medical product and cannabis preparations should be allowed?
WHO / ECDD: Regarding the purity of THC, it was not specified as it varies among medical products and so it is not possible to specify. Regarding the children and young adults’ question, this is outside of our mandate. Regarding new medications, most are in fact relatively old and so there is considerable experience and evidence about such medical use of the substance. About oral administration, the available medications are indeed administered orally but this doesn’t explicitly exclude other types of medications. About the preparations, we are talking about pharmaceutical preparations and it is up to each MS to determine these processes. Usually these products are produced in very regulated, medical contexts.
VNGOC: We are holding a lunchtime event in room C4 to introduce the NGO structures that are relevant to your work.
Chair: We will continue in the afternoon with the nation statements.
AFTERNOON SESSION
Mexico: Some of you might recall I mentioned CBD Therapeutics before. They are doing major work in particular research on diabetics. We have taken note of the concern that other Member States expressed about the term “readily available”. We can say on this is that we cannot lose sight of the possibility to manipulate THC genetically or chemically. So different, more powerful versions will prevail such as the so-called synthetic marijuana. We should be focusing on the toxic element, not Cannabidiol so as our job is, we should understand how they come to the market and what is the damage they are using. Leveraging the knowledge, we didn’t have before that the toxic content is THC not CBD.
European Union: The recommendation 5.6 can be justified with limited availability of crucial medicines. Is there evidence for access? Is there evidence extensive use for Cannabis and Dronabinol?
Pakistan: Threats to human health, particularly youth – we need to duly investigate and some questions needs more clarity. For example, an answer to our question, that it is outside of WHO’s mandate, it should be going to more depths.
Jamaica: The recommendations are a milestone event for the governance of international drug control. This is the first time cannabis is placed under review so we appreciate this opportunity and this discussion. We know why and how the recommendations came about, thanks to the reiteration by the WHO. The questions and detailed responses point us on the direction that the global architecture of drug control needs to be recalculated. We wish to highlight Mexico’s questions at the 4th intersessionals, pointing out the difference between current knowledge of Cannabis in comparison to when the conventions were first adapted. It is our firm belief that a follow-up will strengthen the conventions and allow us to utilize the benefits of the Cannabis plant. It is at the discretion of states to implement stricter controls. The recommendations can serve the purpose of decreasing the illegally cultivated plants. We think we are progressing towards the right direction.
WHO / ECDD: Mexico, there is a lot of interest in the therapeutic uses of compounds of the cannabis plant. We note that but also a number of these compounds are at a preliminary stage of research. Scheduling does affect the availability of a drug for medical reasons. There is no specific evidence regarding cannabis, but many other substances show similar trends in this regard.
Chair: Member States ask for a written summary of all the answers of today.
WHO / ECDD: Yes, we will provide written responses. Thank you all for this stimulating discussion. We are available for any face-to-face or bilateral meetings. Besides the ECDD’s work, we have several relevant inputs.
Chair: Any more interventions? I see none.
INCB Secretariat: This is not an official statement by the Board, I am presenting the assessed implications of the ECDD recommendations. What would be the practical impact of the recommendations? For the daily work of the INCB, there will be more clarity in regards to monitoring. [Response to be shared shortly]
United States: Could some of the aims behind the recommendations be achieved on a voluntary basis by MS? By doing that we would not have to do such a major shift in the legal framework that we have.
INCB Secretariat: UNODC legal services would be able to provide you with more appropriate answers but Member States who do not submit estimates, are established an estimate by the Board based on previous data. This is to enable the country to import the substance. In the ‘71 convention consumption is voluntary but under the ‘61 none of this is optional.
Nigeria: You’ve talked about some of the benefits in terms of your work and controlling substances. Is there anything the INCB will be doing differently if we perhaps vote in favor of this recommendation? Is there anything you are not doing currently? If the INCB is as concerned as very many delegations that the ECDD is not able to authoritatively state that the harmful effects of this substance are not upstaged by the therapeutic value (also not in the first line), how would you respond?
Germany: Just to be very clear, we know article 28 of ’61 excludes industrial hemp (fibres and seeds), does that really mean preparations or tinctures or extracts coming from industrial hemp are not allowed for human consumption?
Norway: I understood that the only use of hemp is limited to fibres and seeds but earlier today, WHO said that CBD containing food products are not controlled if CBD is produces synthetically or it is made from industrial/horticultural hemp. Could you clarify?
INCB Secretariat: We only have cannabis and cannabis resin listed in our forms at the moment, so THC would be reported differently. The quantities are reported separately. When an MS is importing or exporting something like Sativex, there is a conversion factor that needs to be applied to calculate the content THC. Member States report morphine as an example, but they don’t tell us how many kilos of poppy straw was used to produce that morphine. I hope this gives you an idea. The need of estimates and import-export certificates is all ensuring control in that case.
Regarding the abuse ratio, I will let the WHO discuss this as they have a division dealing with substance abuse and they periodically publish studies that document what your to questions touched on. In-depth analysis is provided for medical and non-medical use as well.
Regarding Norway and Germany, we have not looked at the recommendations in detail, but in the preparation of last year’s report, the board clarified that given the current status of the conventions, the cultivation for industrial purposes, it was specifically indicated for fibres and seeds. This was because molecules were not identified yet at the time of writing. In the commentary, as USA pointed out before, the specification for the cultivation of Cannabis is not restricted to seed and fibre but so the question is whether extraction of CBD required the flowering top – which is, according to the convention, is controlled as cannabis.
United States: In reviewing the official record of CND, it appears that originally there was language that limited to fibres and seeds but it was withdrawn and was later inserted in parentheses. That made us question what are the real limitations? Our other questions is about the obligations of MS under the single convention as opposed to the ’71. Is this not something INCB can take on? We are accustomed to relying on INCB for guidance on such things.
INCB Secretariat: I sympathise with the concern of the USA. The Board has reviewed those documents and indeed there is this unclarity that only drafters of the amendment of the convention really know. We are reading what is in the text, the treaty is the fundamental text, the commentary is an additional crutch, but it is not a definitive text. The MS signed the treaty and not the commentary. Regarding fibres and seeds, it seems the limitation is only regarding that. Again, I am as puzzled as you are, but we have to work with the language that is there. Even before ECDD looked at the issue, we had questions about CBD reporting. We used to encourage Member Statesto report under the ’61 convention as cannabis. Some importers might not even know the origin of the THC is plant-based, synthetic or mixed… It is for the MS to carefully consider the ECDD recommendations.
Nigeria: What we wanted to know is that this new approach, if the recommendation passes, how will it improve the work of INCB in terms of controlling this substance? We saw the INCB’s opinion about the lack of sufficient evidence and we feel more aligned than to the WHO.
INCB Secretariat: WHO has conducted their work strictly along their mandate. It is not for INCB to provide opinions on this. I would be very careful with this. I am not in a position to respond to this.
In regards to the consequences to our work, it is a mix of elements. All the substances under one convention will facilitate monitoring and control. On the other hand, cultivation needs to be clarified. People might be under the impression that since CBD is not scheduled, Cannabis cultivated for CBD needs no control. We pointed out that if a controlled substance can be extracted from the plant, they need to report on it – similar to poppy.
Chair: I see no further questions. Thank you INCB. I invite the UNODC to answer their questions.
UNODC: Our answers will be provided in a written form in the coming days.
Responding to general questions: We are not able to reply in the most comprehensive matter about the national impacts; it would be different for each member state. The 61 and 71 conventions contain several similar resolutions, we understand that reference to differences might be of assistance. The 61 has a stricter system than the 71. The 61 addresses manufacture and importation, it deals with licensing – we will provide an illustrative report in writing that we hope will be useful. The impact of the recommended changes will differ from country to country. 25 years of transitional time as per 61. The 71 offers signature ratification of exceptions. The possibility of reservations for traditional use is available but have not been claimed by any member state. Our reply regarding the definition of Cannabis resin repeats the wording of the convention. According to the 61 commentary, no part of the plant is excluded as the source of the resin. The WHO specifically noted that they need notification. We would refer the scientific justification to the WHO. As a matter of practice, recommendations are voted on as they are presented. We vote on each recommendation separately. In case of conditionality, they will follow each other in a logical manner. If 5.2.1 doesn’t go through, then 5.3.1 will not be put to a vote. If 5.2 is yes then we go ahead with 5.3 and that could be adopted or not.
Regarding Article 28 of the 1961 Convention, the scope of this would not be modified. It would continue to be applied for the cultivation of the plant. Only preparations described in the footnote would be excluded. Non-psychoactive preparations would also fall outside of the scope. Some country follow a more literal interpretation and they would likely continue as such, should the recommendations would be adapted.
Regarding the footnote related questions, we refer back to the WHO.
Chair: Thank you. I open the floor for interventions.
Community Alliances for Drug Free Youth (CADFY): [This intervention will be added shortly]
Secretary: I would like to remind delegations that we are in the midst of the meetings of subsidiary bodies. So, as established practice, regional meetings will produce their own reports that will be brought back to the commission for consideration in March. A briefing on the work of the CND in New York will take place on the 2nd of October.
Chair: If there is nothing further, I will conclude the meeting for today.